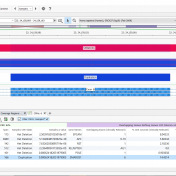
Introduction: Malignant Rhabdoid tumors (MRT) are among the most aggressive and lethal forms of infant and child cancer (1). These tumors are characterized by an unusual combination of mixed cellular elements similar to but not typical of teratomas and can originate at any anatomic location. When MRTs are present in the brain, they are called atypical teratoid/rhabdoid tumors (AT/RT), which… Read more »
Abstract Before assessing the clinical significance of a somatic mutation, one must determine if the mutation is likely to be a driver mutation (i.e. a mutation that provides a selective growth advantage, thereby promoting cancer development). To aid clinicians in this process, VSClinical provides an oncogenicity scoring system, which uses a variety of metrics to classify a given somatic mutation… Read more »
Thank you to everyone who joined me for yesterday’s webcast, I hope you all enjoyed it. If you missed the live event and are interested in knowing what we talked about, good news, you can watch the recorded version right here! There were so many great questions asked during our Live Q&A that I was unable to answer all of… Read more »
Our Support Team curates a variety of tutorials to help orient new users to the capabilities of VarSeq. We are happy to announce the team’s new release of the trio tutorial that places emphasis on using the ACMG guidelines. This tutorial gives insight into the proper setup of pedigree structure as well as detailed descriptions of the filter containers and… Read more »
Introducing Drugs & Trials for Cancer Diagnostics VSClinical offers enormous simplicity and consistency in evaluating biomarkers and providing treatment options. Last month, we announced our newest feature to now include the automated collection of relevant clinical trials. The new capability was unveiled in our “Introducing Drugs & Trials for Cancer Diagnostics” webcast with Nathan Fortier, Ph.D., Director of Research, showing… Read more »
Customizing VSClinical ACMG Guidelines Workflow Part 2 In the first part of this series, we covered how VarSeq provides customization of the clinical analysis workflow process. VSClinical’s various customization parameters within the ACMG Guidelines workflow includes the choice of how the internal knowledge base of previous variant interpretations are stored and what considerations go into this choice. In this blog, we… Read more »
When interpreting a variant using the AMP/ASCO guidelines for somatic variant interpretation, clinicians must determine whether the variant can be considered a biomarker that affects clinical care by predicting sensitivity, resistance, or toxicity to a specific therapy. Such a determination requires the investigation of multiple evidence sources, including clinical trials, FDA approved therapies and peer-reviewed studies. Unfortunately, strong evidence linking… Read more »
Clinical labs offer a unique and sophisticated product that is performed repeatedly with high standards of quality. VarSeq was developed to provide labs with the customization required for clinical genetic tests in a repeatable workflow. On top of this, VSClinical offers additional parameters and choices that can be made when designing the test workflow. In this blog series, we will… Read more »
If you have watched this blog over time, it would be no surprise that Golden Helix invests a lot in curating genomic annotations for use with our clinical and research analysis products. Often, we spend considerable time on the attention to detail necessary to ensure the best experience for any data source by cleaning, normalizing, documenting and then distributing it through our data annotation server. Many annotations… Read more »
Our tutorials are a popular resource for prospective users. We have added a tutorial to help introduce customers to the ease and utility of the ACMG Guidelines incorporated in VSClinical. The ACMG Guidelines are a collection of 33 criteria that are used to determine a variant’s level of pathogenicity. VSClinical and VarSeq make it easy for users to sort and… Read more »
We have recently added a tutorial to help introduce customers to the ease and utility of the AMP Guidelines incorporated in VarSeq’s VSClinical package. The AMP Guidelines allow users to sort through available clinical evidence in a streamlined fashion to arrive at final classification and interpretation and then transfer that information into a clinical report. And the AMP Guidelines also… Read more »
This month’s webcast delves into VSWarehouse with a focus on our new capability of storing somatic variant projects and catalogs built for the AMP Guidelines within VSClinical. If you didn’t have a chance to join us for the live event, please enjoy the video recording below. Previous webcasts have gone into great detail on the features and processing of somatic… Read more »
The Golden Helix team is excited to be headed to Baltimore for the AMP 2019 Meeting, and we hope to see you there! You will find our team at booth #2856. Swing by or schedule a time to talk with us one on one about how Golden Helix’s platform is redefining next-gen sequencing analysis. Our team would love to hear… Read more »
As our team returns from another successful ASHG conference, I would like to reflect on the great memories, connections, and future plans that were made at this meeting. First, I will start by thanking everyone involved with the superb planning and execution of the conference. We are thankful to have this opportunity to connect with our customers, partners, and friends… Read more »
The common approaches to detecting copy number variants (CNVs) are chromosomal microarray and MLPA. However, both options increase analysis time, per sample costs, and are limited to the size of CNV events that can be detected. VarSeq’s CNV caller, on the other hand, allows users to detect CNVs from the coverage profile stored in the BAM file, which allows you… Read more »
Before examining the clinical evidence associated with a specific mutation, a clinician must establish that the variant is likely to be a driver mutation which generates functional changes that enhance tumor cell proliferation. Our recent blog series “Following the AMP Guidelines with VSClinical” briefly mentioned how the oncogenicity scoring system in VSClinical could be used to automate and assist the… Read more »
Gene Fusion Background Gene fusions are hybrid genes that result from translocations, interstitial deletions, or chromosomal inversions that can lead to constitutive gene activation and result in increased or abnormal protein production. Increased or abnormal protein production subsequently can play an important role in tumorigenesis and thus identifying and evaluating this type of biomarker is important in the cancer workspace…. Read more »
VSClinical users can interpret and report genomic mutations in cancer following the AMP guidelines which we’re demonstrating in this “Following the AMP Guidelines with VSClinical” blog series. Part I introduced the hands-on analysis steps involved in creating a high-quality clinical report for targeted Next-Generation Sequencing (NGS) assays. We reviewed sample and variant quality, including the depth of coverage over the target regions by the sequencing performed for each sample. Now, we are ready to… Read more »
In the world of genomics shaping precision medicine in oncology, the limiting factor is the time-to-sign-out of a fully interpreted molecular profile report. There are many components of the entire testing process that add to the turn-around time of each test. Many of these steps, such as sample prep, sequencing, and automated secondary analysis, are bounded and consistent in their time requirements. The hands-on… Read more »
With the increasing knowledge of mutations involved in cancer, it is imperative to have a tertiary analysis pipeline that provides users with the most up to date information on somatic mutations. VSClinical’s Cancer Add-On does just that and more; with this feature, users can investigate and report on SNVs, indels, CNVs, gene fusions, and considerations for wild type genes in… Read more »