Clinical Variant Analysis for Cancer – Applying AMP Guidelines to Analyze Somatic Variants Detecting cancer at an early stage can make it much more treatable. Developing tests and making them clinically actionable is crucial to beat this disease. This eBook covers the state-of-the-art gene panel tests for cancer. Of course, there is more that can be done. The field is… Read more »
We are upgrading all VSClinical +Cancer Add-On purchases to a 15-months license! One license of VSClinical +AMP Guidelines – $17,995* Additional Users: $8,995* The individualized nature of tumors requires genomic testing for providing the best outcomes for patients. Next-Gen Sequencing enables the detection of small mutations, copy number changes, and common fusions affordably and with high precision. However, the interpretation… Read more »
Clinical Variant Analysis for Cancer – Applying AMP Guidelines to Analyze Somatic Variants Somatic variants can manifest in different ways: Single Nucleotide Variants Indels Fusions and Copy Number Variations There is a difference between the interpretation of germline and somatic variants. The former is exclusively focused on establishing the level of pathogenicity vis a vis a particular disease. In contrast… Read more »
Clinical Variant Analysis for Cancer – Applying AMP Guidelines to Analyze Somatic Variants As described in my eBook “Genetic Testing for Cancer,” any bioinformatic pipeline for cancer ultimately calls variants based on the aligned reads that the sequencer generated. Variant calling is the process of reviewing a sequence alignment, typically in the form of a BAM file, to identify loci… Read more »
Clinical Variant Analysis for Cancer – Applying AMP Guidelines to Analyze Somatic Variants Analogous to the ACMG guidelines for germline mutations, the Association for Molecular Pathologists (AMP) has issued guidelines to assess and report on somatic variants. The key paper in this area was published by Li et. al (2017) with the title “Standards and Guidelines for the Interpretation and… Read more »
Clinical Variant Analysis for Cancer – Applying AMP Guidelines to Analyze Somatic Variants Precision Medicine uses genetic information from individual patients. This may include the following areas: Diagnosis Treatment Prevention Specifically, in the cancer space, data derived from Next-Gen Sequencing (NGS) is used to diagnose and prognose diseases, select a targeted therapy and potentially evaluate the suitability of a patient… Read more »
Applying AMP Guidelines to Analyze Somatic Variants Today, I am thrilled to share with you the launch of a brand new eBook titled “Clinical Variant Analysis for Cancer – Applying AMP Guidelines to Analyze Somatic Variants”. We would happy to send you a complimentary copy which can be requested on our website here. The clinical utilization of Next-Gen Sequencing data… Read more »
Yesterday, I released a new version of my eBook, “Genetic Testing for Cancer – Third Edition”. We would be happy to send you one! To download a complimentary copy, please submit a request on our site here. In 1914, the German cytologist Theodor Boveri coined the phrase “Cancer is a disease of the genome.” At this time, his ideas were… Read more »
This webcast generated a lot of great questions about the content covered in the video above. I have summarized our Q&A session below and included some questions I didn’t have time to answer during the live event. If you have any further questions, reach out to us at info@goldenhelix.com! Q: Can I upload my existing classifications into a consortium source?… Read more »
Our “Processing Hereditary Cancer Panels in VarSeq” webcast was a great lesson for viewers to learn more about the functionality of our software. If you didn’t have a chance to join us for the live event, you can watch the recording on our site here. Q: What is the best place to get clinical interpretations of breast cancer variant interpretations?… Read more »
CIViC The Clinical Interpretations of Variants in Cancer, better known as CIViC, is an open access open source, community-driven web resource available to all VarSeq users. Nature Genetics published an article that states, “CIViC accepts public knowledge contributions but requires that experts review these submissions”. Fundamentally, the focus behind CIViC is to make sure the variants contained in the database… Read more »
VarSeq enables breakthrough discoveries in cancer diagnostics by supporting gene panel testing and whole exome and genome analysis. We wanted to share our Cancer Gene Panel tutorial which covers a basic gene panel workflow with an emphasis on adding, modifying and manipulating filter chains. This tutorial will start with creating a new project from an empty project template, importing data, creating… Read more »
While clinical assessments of germline mutations have been collected in ClinVar under the stewardship of the NCBI and the collaborate effort of many testing labs, the same type of resource has been missing for mutations that could informal clinical care in Cancer. Or at least, that is what I thought until I started to work with CIViC. With the stewardship of… Read more »
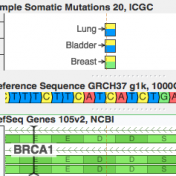
ICGC’s database is now available For quite a while, COSMIC has been synonymous with the catalog of “known somatic mutations”. It is the ClinVar of cancer mutations and invests heavily in “expert curation” (having human experts read papers and pull out references to published somatic mutations). But it turns out there is a new kid on the block, and he… Read more »
Recently, we were excited to find a new example data set for cancer gene panels. We have included this example data in the latest e-book by Dr. Andreas Scherer, Genetic Testing for Cancer as well as in the latest cancer webcast . This data is from Illumina’s MiniSeq sequencer and the TruSight Cancer panel. The BAM and VCF files for three samples… Read more »
In 1914, the German cytologist Theodor Boveri coined the phrase “Cancer is a disease of the genome”. At this time, his ideas were as equally revolutionary as they were highly contested. Fast forward. More than a hundred years later, Next-Generation Sequencing effectively permits a highly sensitive analysis of cancer cells. It can help us to understand mutations associated with cancer… Read more »
Wednesday, March 2nd 12:00 pm EST Clinical labs must have the ability to go from a collection of samples and associated variants to a professional report documenting a short list of clinically relevant variants. Cancer Gene Panels are a common clinical application for genetic tests. In this webcast we will show how VarSeq and VSReports can be used to go… Read more »
Last week we conducted a webcast on “Cancer Gene Panels”; you can find the recording here. We had some excellent questions which we answered during the webcast and a few more that we didn’t get to in the allotted time. Please find answers to those questions here: 1. Are Cancer Gene Panels just another stepping stone on the way to… Read more »
In 1914 the German cytologist Theodor Boveri coined the phrase “Cancer is a disease of the genome”. At this time his ideas were equally revolutionary as they were highly contested. Fast forward. More than hundred years later, Next-Generation Sequencing effectively permits a highly sensitive analysis of cancer cells. It can help us to understand mutations associated with cancer development and… Read more »
Dr. Folefac Aminkeng is a Postdoctoral Fellow at The Centre for Molecular Medicine and Therapeutics (CMMT) at the University of British Columbia in Vancouver, BC, Canada. He utilizes GWAS studies to identify single-nucleotide polymorphisms (SNPs) that might be associated with serious adverse drug reactions (ADRs) in cancer therapeutics. The field of pharmacogenomics—how one’s genetic makeup affects drug response—has grown exponentially… Read more »