When interpreting a variant using the AMP/ASCO guidelines for somatic variant interpretation, clinicians must determine whether the variant can be considered a biomarker that affects clinical care by predicting sensitivity, resistance, or toxicity to a specific therapy. Such a determination requires the investigation of multiple evidence sources, including clinical trials, FDA approved therapies and peer-reviewed studies. Unfortunately, strong evidence linking specific genetic biomarkers to FDA-approved therapies only exists for a small number of cancers. Thus, most variants require an exploration of clinical practice guidelines, peer-reviewed literature, and large-scale cancer mutation databases to effectively assess the clinical significance of a given mutation.
We are excited to announce that VSClinical will soon be assisting this process by providing clinicians with an all-new functionality for exploring clinical therapies relevant to the patient’s specific tumor type and biomarkers.
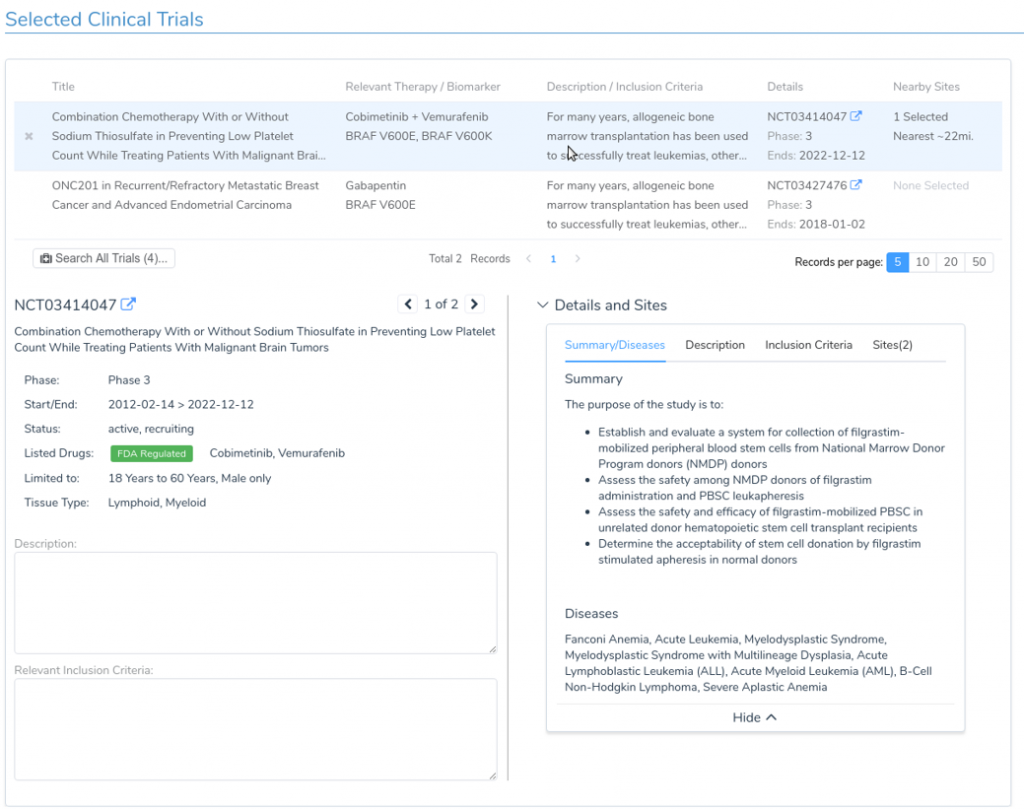
The incorporation of Drugs & Trials Annotations into the VSClincal AMP Guidelines Workflow will allow the clinician to quickly examine relevant FDA approved therapies and clinical trials based on the specific mutations present in the sample. For each targeted therapy, the user is provided with a detailed description of the treatment, along with information on relevant publications. Additionally, for relevant clinical trials, the clinician is provided with important information including inclusion criteria, the trial’s recruitment status, and contact information. VSClinical makes it easy to incorporate this information into the clinical report, with built-in access to publication abstracts and automatic citation management based on PubMed IDs.
Data Sources
Two data sources primarily power this functionality. The first is DrugBank, a comprehensive online database of drugs and drug targets. This database provides rich information about targeted cancer therapies along with associated genes and genetic variations. By leveraging this data source, we can provide the user with detailed and up-to-date information on available FDA-approved targeted therapies and related peer-reviewed literature. The second data source leveraged by our Drugs and Trials tool is the NCI clinical trial database. For each NCI-supported clinical trial, this database provides a detailed description of the therapy along with information on associated tumor types, biomarkers, and inclusion criteria. By leveraging these rich data sources, VSClinical allows the clinician to quickly access the relevant clinical evidence for a given biomarker using the latest information on available targeted therapies.
Learn more by watching the on-demand recording
This webcast explores this new incorporation of Drugs & Trials Annotations in VSClinical’s AMP Workflow covering:
- Identification of relevant clinical evidence for drug sensitivity and resistance based on patient biomarkers and tumor type
- Review of clinical trial information including inclusion criteria, trial status, and contact information
- Management of citations associated with relevant, targeted therapies
- Evaluation of a biomarker’s clinical evidence tier based on available evidence for drug sensitivity and resistance