As clinical genetic tests have been adopted as a critical enabler of precision medicine, the number of tests offered by clinical labs and the volume of tested patients has grown by orders of magnitude in the past five years. The Gene Testing Registry, managed by the NIH, documented a rise from 13,000 to 60,000 tests offered in the US market between 2013 and 2018. These cover many medical needs, including diagnosing rare diseases, predicting lifetime risk of inherited diseases such as hereditary cancer, and profiling the genome of tumors.
Each of these tests requires highly trained medical professionals to follow complex workflows and interpret the results. Their time is often the constrained resource of genetic labs. Golden Helix has developed an end-to-end solution for these different clinical genetic tests while providing use-case-specific workflows following industry-standard guidelines that efficiently use the valuable time of lab personnel.
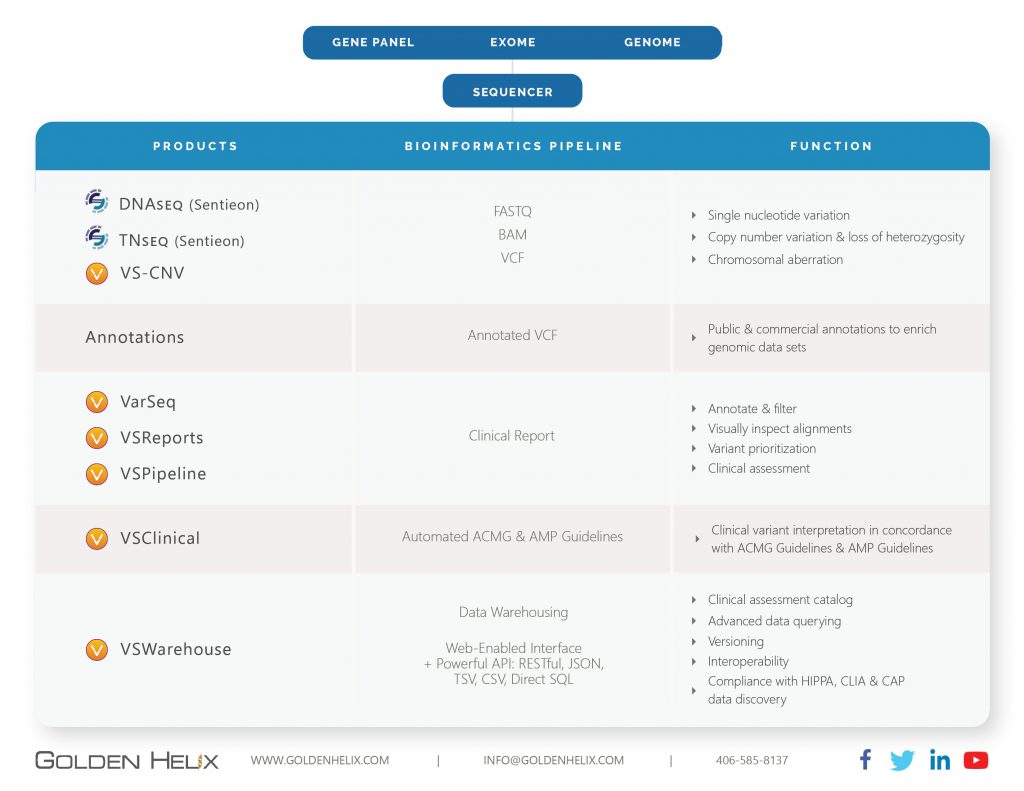
Comprehensive Architecture for Clinical Genetic Testing
The power of Next-Generation Sequencing is the detection of every unique and individual mutation in a relevant set of genes through an affordable assay that is no more complex to run than other laboratory tests. But while the raw production of the multitude of short sequencing “reads” covering your targeted genes is relatively straightforward, the downstream analytics and clinical interpretation of the known and potentially novel variants in a given patient requires an enormous amount of software complexity. Golden Helix has built a complete end-to-end bioinformatics pipeline that is designed to receive the raw sequencer output and take it all the way to clinical reporting. Alongside this, we have created automation capability for high throughput labs, as well as an extensive data warehousing capability that allows the capture and querying of the entire lab output.
This complete end-to-end architecture sets Golden Helix apart in the marketplace. It supports the thorough analysis of the per-sample data coming out of Next Generation Sequencing machines. It takes the analysis to the end-product of the clinical test: the genetic test report. Finally, it stores all the intermediate and final data in a repository that allows integration to laboratory and hospital record systems through standard protocols and APIs.
Secondary Analysis: Here, we provide the unique ability to analyze genomic data in regards to Single Nucleotide Variations and Structural Variations. Via our partnership with Sentieon, we provide a highly performant secondary pipeline that includes alignment and variant calling on par with GATK and MuTect2 at much-improved speed levels. Our product VS-CNV is capable of detecting CNV events starting at the exon level and all the way to aberrations of an entire chromosome.
Tertiary Analysis: VarSeq and VSClinical are covering all clinically relevant workflows for the filtering and annotating of genomic data. For example, it supports gene panels, trios, single exome, and whole genome workflows. With a single click, users can generate a clinical report that integrates the specific findings with annotation sources. VSClinical provides specialized workflows to answer the variant scoring criteria of the ACMG and AMP guidelines or the interpretation of germline and somatic variants, respectively. Reports can be customized to meet the requirements of each clinical test, while the interpretations of variants are stored in a laboratory knowledge base that reduces the work done on each new sample.
Data Warehousing: VSWarehouse captures the artifacts of the bioinformatics pipeline. Via powerful APIs, the product connects to other lab and hospital systems such as EPIC or Cerner’s Millennium. Moreover, it allows you to answer the following questions efficiently:
- Have I seen this variant before in my clinical practice? If so, was it included in any clinical report?
- Has the categorization of any variant that I reported on changed (e.g., from ‘unknown’ to ‘pathogenic’)?
- It allows you to version the clinical analysis conducted by lab work, including the annotation sources that have been used during the tertiary analysis. This is a key capability during discovery should your lab or hospital be involved in legal disputes.
Our software stack is deeply integrated. This is a major advantage over any software architecture consisting of various disparate solutions of other vendors or homegrown applications. The high degree of integration brings enormous speed and performance advantages and flexibility since we have been able to optimize data formats and leverage multi-threaded code algorithms across the various products.
Automation While Maintaining Flexibility
While it is possible to provide a commercial solution that performs one fixed genetic testing workflow in an automated fashion, the power of the Golden Helix solution is the enormous flexibility to match the exact filtering, annotation, reporting and workflow parameterization that allows a laboratory-developed test to reflect the laboratory expertise configured into each testing procedure. Once the Golden Helix workflow is fully configured, it can be locked down and run on-premise, behind the institutional firewall, and without requirements for internet resources until the laboratory is ready to update that pipeline to a new assay or software version. In total, the Golden Helix clinical testing software architecture provides:
- Customizability: Design the exact workflow for the clinical test at hand, branded and representative of the laboratory standard operating procedure and integrated into existing laboratory systems
- Automation: With VSPipeline, we have developed the ability to automate the entire pipeline to increase throughput while going from the FASTQ to Clinical Report. Along with saving time, this automation also minimizes the potential for human error.
- Security and Deployment Flexibility: With on-premise software with locally run algorithms and annotations, the entire analysis happens in an un-metered secured environment. Whether you deploy to cloud instances, internal compute clusters, or commodity lab resources, our software runs in your environment on any OS and network configuration.
Golden Helix is pleased with its global market adoption, with references in the clinical NGS-based market across the globe. Our work has been recognized by multiple media outlets, such as CIO Review and more recently being named part of the Inc 5000. Please get in touch with us if you would like to learn more about how we can help your lab maximize the potential of your NGS tests.