Webcast Recap
In the recent webcast “Exploring New Features and Clinical Reports in the ACMG Guideline Workflow”, Gabe and I took viewers through an evaluation with CNVs and SNVs according to the ACMG Guidelines where we generated and customized a clinical report. Along the way, we highlighted many new features that will soon be available in the upcoming VarSeq release.
Below are the features and updates that were discussed:
- New and improved ACMG Classifier algorithms
- Improved assessment catalogs for saving classifications and interpretations
- Updated Project Options interface for creating patient evaluations
- Now offering three new word-based clinical report templates
- Inclusion of additional sections and features to the clinical reports
- Enhanced report customization capability
If you were not able to attend the live webcast session, no worries! You can watch the on-demand recording of the webcast right here.
Featured Questions
Q: Can the Microsoft Word-based features work with other operative systems where Microsoft Word is not used, i.e. GNU-Linux, LibreOffice writer, WPS, etc.?
A: Absolutely! We are not dependent on Word existing to create the docx file. We are creating a docx file and asking the operating system to open that. Specifically, in Linux, the docx file opens in the free LibreOffice suite and is able to display this information because it is an open standard format. So these word-based reports are functional on Windows, Mac, and Linux. Also as an additional note, these reports can be generated with our command-line VSPipeline mode as well.
Q: Does Golden Helix software do the whole analysis process from aligning the raw FASTQ files to generating the report?
A: Yes, Golden Helix software offers a full-stack solution. We have a partnership with Sentieon, which can perform the alignment and variant calling, creating the VCF and BAM files. The VCF and BAM files can then be imported in VarSeq for variant annotation, filtering, and interpretation. Eventually, this information can be used to create a clinical report. Our Warehouse solution provides researchers and clinicians access to this information and to view previous findings.
Q: Can you use the reporting feature in another language? Can every single aspect of it be translated?
A: This is definitely possible! The report template itself is just a mix of the fixed language for the report, which is then used to fill in the information for the current sample in the evaluation. Both the fixed text and the interpretation text from your variant analysis workflow can be written in your preferred language. Since there is also a flexible programming interface, you can create auto-generated summary paragraphs with your language of choice.
Q: Will the old report templates and reporting capabilities still be available in the new release?
A: Absolutely! The old reporting capability with VSReports is still available within the software. This link will refer you to the tutorial that provides information on the old reporting system and how to make some minor report customizations. If you have already customized a report template from the old VSReports system and wish to use the new word-based report templates, we can work with you to transfer those customizations to the word-based system. The new Mendelian Disorder report was designed to be very similar to the default ACMG template report in the VSReport system to make the transition easy for most use cases.
Q: Can my clinical reports be integrated into a LIMS system?
A: Yes, there are number of options to transform the data and integrate it into the LIMS system. Generally, some transform of the data from raw structured data format that is used as input to the Word template is sufficient (see final question below). There are also possibilities to automate this process with VSWarehouse or through a custom UI built into VarSeq using the existing VSReports system. In general, we can find the right solution that fits a given laboratory configuration with a quick call with one of our field application scientists!
Q: Is there a director-level sign off for the clinical reports?
A: Yes, the director level sign off is available in the report tab of the ACMG Guideline workflow within VSClinical. Once a report is signed out, changes cannot be made to the scored criteria or interpretation.
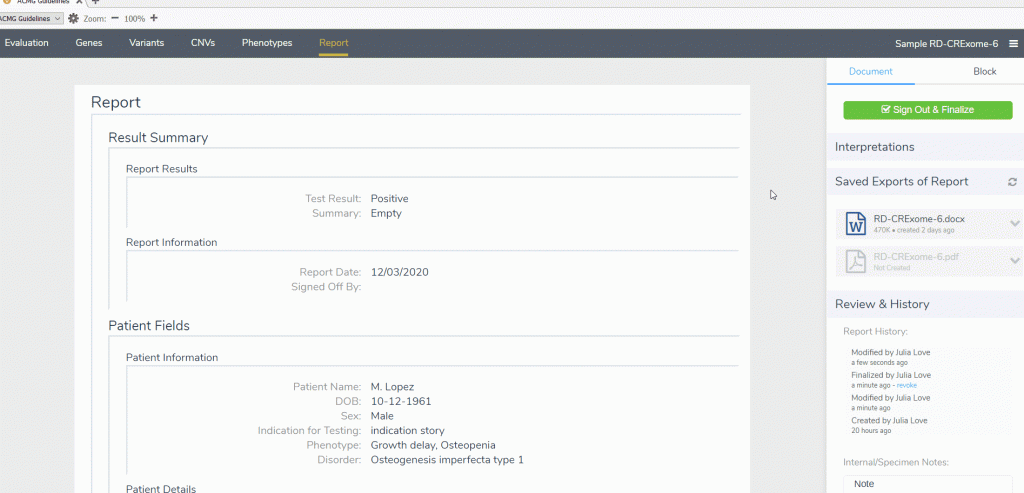
Q: Can reports be rendered into formats other than a Microsoft Word document?
A: Most Definitely! From VarSeq, reports can be rendered as word documents and PDFs. The word doc can be manually converted to a PDF or automatically converted using our cloud service.
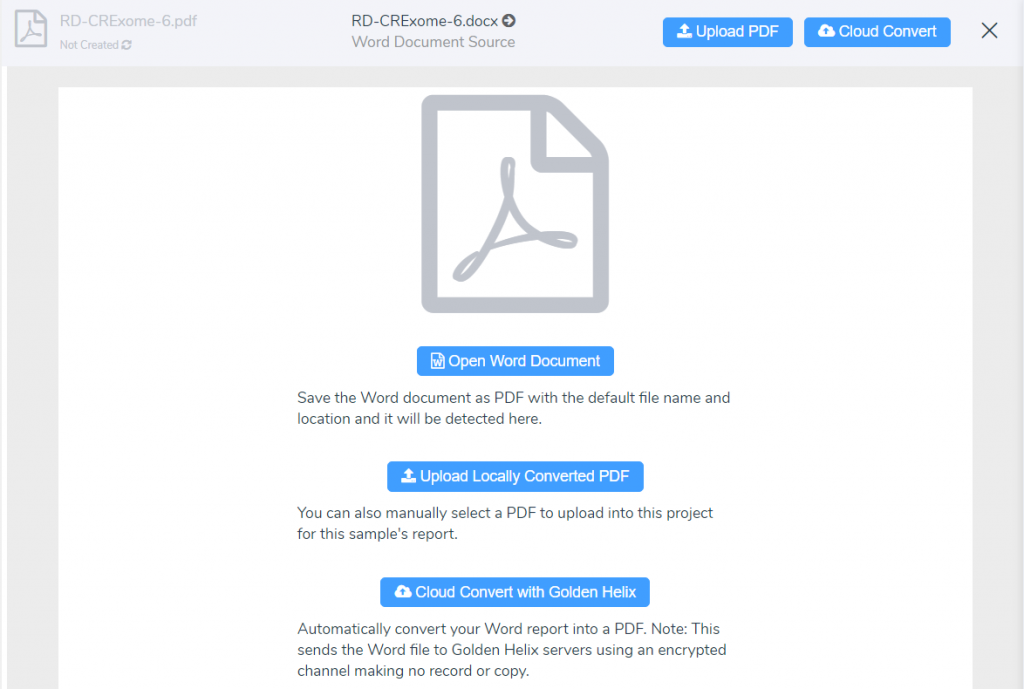
If there is another format that you wish to use for the clinical reports, the raw structured data is available as the JSON file. This can be used to manually transform the report into whatever format you would like, such as XML, HTML, or plain text for example.
Thanks to all who attended this webcast, ‘Exploring New Features and Clinical Reports in the ACMG Guideline Workflow’! We are looking forward to joining you for another great collection of webcasts in the New Year. Make sure you are on our mailing list to receive our webcast invitations or find more information here. Feel free to check out some of our other blogs that always contain important news and updates for the next-gen sequencing community.