Clinical Variant Analysis for Cancer – Applying AMP Guidelines to Analyze Somatic Variants
Somatic variants can manifest in different ways:
- Single Nucleotide Variants
- Indels
- Fusions and
- Copy Number Variations
There is a difference between the interpretation of germline and somatic variants. The former is exclusively focused on establishing the level of pathogenicity vis a vis a particular disease. In contrast to that, when we assess the clinical implication of a somatic variant, we are interested in the following:
- Sensitivity: Does a particular variant imply the sensitivity to a particular drug or treatment.
- Resistance: Here, we might establish that certain drugs are not effective.
- Toxicity: In this case, we might find that a certain biomarker implies that a certain drug has adverse or toxic side effects.
The AMP guidelines suggest to cluster the available clinical evidence in four levels:
- Level A: This is the highest level of available evidence. Biomarkers in this category are clearly established to either create a definitive tumor response for a specific tumor type or are known to indicate resistance of the tumor based on US-FDA or other professionally accepted guidelines.
- Level B: There is still very strong evidence of a predicted tumor response or resistance based on numerous large studies. Experts agree on the findings, and the assembled body of knowledge is consistent in the clinical interpretation of a given biomarker.
- Level C: Biomarkers in this category point towards efficacy or resistance in a different type of tumor. Clinicians use Level C to recommend off-label use of a drug. Sometimes they are used to recommend the inclusion of a patient in a clinical trial.
- Level D: In this category, evidence from pre-clinical studies or other smaller studies on a smaller scale is collected. This is generally seen as the weakest of all pieces of evidence that can yield a clinical decision.
The available clinical evidence is then sorted into four tiers that describe the clinical impact of any given variant (see fig 11).
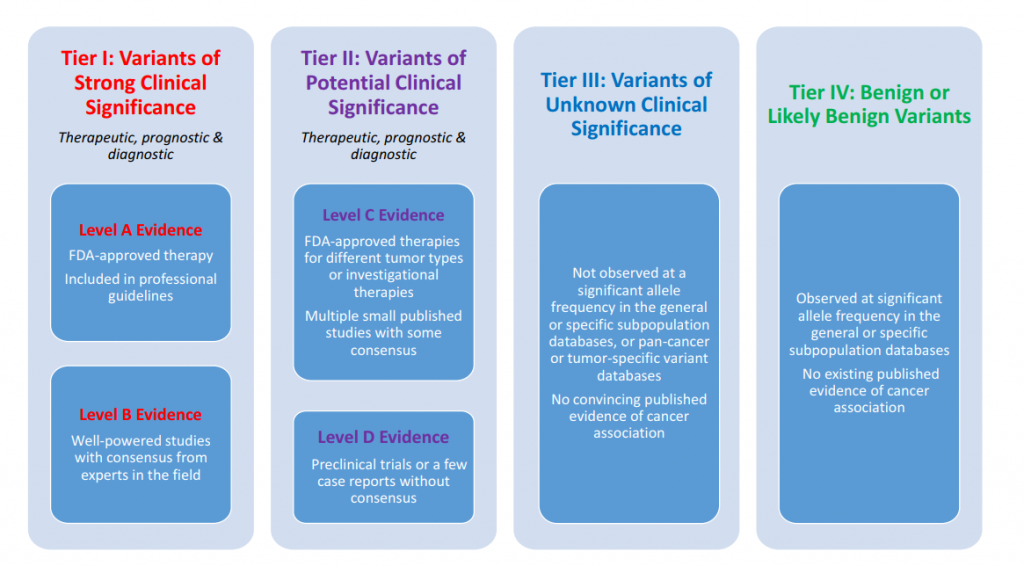
Tier I Variants
Here we have clear evidence of therapeutic, diagnostic, and or prognostic value. In this tier, we are mainly interested in variants with a predicted response or resistance to FDA approved drugs or others are documented in professional guidelines for a specific tumor. We include only Level A and B evidence in this tier. Let’s look at a few examples.
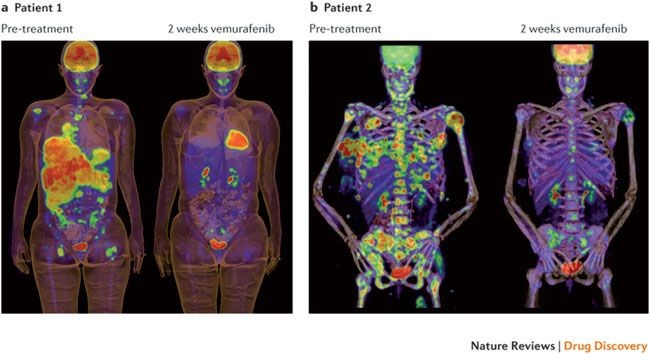
One of the most well-known cancer mutations, BRAF V600E is predictive for a positive response for the drug vemurafenib in melanoma (see fig 12). Therapeutically, this would be a valid and likely choice based on the observed biomarker.
If you wish to continue reading the eBook, I invite you to download a complimentary copy of my eBook. You can do so by clicking on the button below.