Thank you for attending the webinar focused on implementing VarSeq and VSClinical for family-based workflows. If you would like to use the webinar as a reference or were not able to attend, you can access it using the following link to view ‘Family-Based Workflows in VarSeq and VSClinical. Here is a brief recap of what we discussed: This webinar demonstrated… Read more »
Didn’t catch the webcast live? No worries! We cover ‘VSClinical: A Complete Clinical Workflow Solution’ Q&A’s in this blog post. The webcast, ‘VSClinical: A Complete Clinical Workflow Solution’ demonstrated how solutions provided by Golden Helix can be implemented to cover all requirements of a clinical workspace. Specifically, this webcast focused on a detailed workflow from a bioinformatician, geneticist, and lab… Read more »
Webcast Recap In the recent webcast “Exploring New Features and Clinical Reports in the ACMG Guideline Workflow”, Gabe and I took viewers through an evaluation with CNVs and SNVs according to the ACMG Guidelines where we generated and customized a clinical report. Along the way, we highlighted many new features that will soon be available in the upcoming VarSeq release…. Read more »
In the webcast, Evaluation of Copy Number Variants with VSClinical’s New ACMG Guideline Workflow, we discussed how VSClinical implements Section 4 of the ACMG guidelines. Specifically, we focused on integrating literature and publications to assess the pathogenicity of a CNV event when there was a lack of dosage sensitivity information. One of the primary pieces of evidence for evaluating genes… Read more »
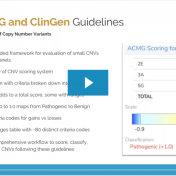
Our previous webcast from VP Gabe Rudy in September exposed us to some fundamentals of this years’ updated Technical standards for the interpretation and reporting of constitutional copy-number variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics (ACMG) and the Clinical Genome Resource (ClinGen). This recent webcast was dedicated to breaking down these new guidelines… Read more »
In our recent webcast announcing the upcoming release of VarSeq VSClinical and the implementation of the ACMG guidelines for NGS CNVs, we had a number of live questions we didn’t get a chance to cover at the end of the presentation. I will follow up on those questions in this blog post. But first, if you didn’t get a chance to join us for… Read more »
Thank you to everyone who joined our webcast, “Whole Genome Trait Association in SVS.” If you missed the live event and are interested in knowing what we talked about, you may access the recorded event below: Our Live Q&A generated a lot of great questions. Unfortunately, we were unable to answer them all, but we have compiled some of the… Read more »
Abstract Before assessing the clinical significance of a somatic mutation, one must determine if the mutation is likely to be a driver mutation (i.e. a mutation that provides a selective growth advantage, thereby promoting cancer development). To aid clinicians in this process, VSClinical provides an oncogenicity scoring system, which uses a variety of metrics to classify a given somatic mutation… Read more »
Thank you to everyone who joined me for our latest webcast, “Next-Gen Sequencing of the SARS-CoV-2 Virus with Golden Helix.” If you missed the live event and are interested in knowing what we talked about, you may access the recorded event below: Our Live Q&A generated a lot of great questions. Unfortunately, we were unable to answer them all, but… Read more »
Golden Helix ships a variety of templates that are designed to provide a starting point for users to evaluate variants in VarSeq. Naturally, as users become more familiar with the software, there is a desire and necessity to tailor the template design to accommodate a more thorough variant analysis. To add to these template customizations there are several algorithms and… Read more »
When interpreting a variant using the AMP/ASCO guidelines for somatic variant interpretation, clinicians must determine whether the variant can be considered a biomarker that affects clinical care by predicting sensitivity, resistance, or toxicity to a specific therapy. Such a determination requires the investigation of multiple evidence sources, including clinical trials, FDA approved therapies and peer-reviewed studies. Unfortunately, strong evidence linking… Read more »
Genetic testing labs deal with personal data in categories with the highest level of security requirements: personal identity and medical records. Given the liability and risk associated with a breach of this secure information, it is not surprising that many labs and institutes that aggregate genomic data prefer, if not require, on-premise analysis and storage solutions. Golden Helix is in… Read more »
SVS is a project-oriented program that manages and analyzes genomic datasets. This webcast statistically and visually explores the relationships among genetic variants within a cattle dataset. Even further, this webcast evaluates genotypes with corresponding phenotypes to assess how well a model can predict a phenotype of interest. Starting with genotypic data from the microarray and the recorded phenotypic data for… Read more »
This month’s webcast delves into VSWarehouse with a focus on our new capability of storing somatic variant projects and catalogs built for the AMP Guidelines within VSClinical. If you didn’t have a chance to join us for the live event, please enjoy the video recording below. Previous webcasts have gone into great detail on the features and processing of somatic… Read more »