Dr. Auber is the team leader for the molecular genetic diagnosis of hereditary diseases at the Institute for Human Genetics at Hannover Medical School (MHH). MHH is one of the largest hospitals in northern Germany, with one of the largest outpatient clinics for individuals and families dealing with hereditary cancer and predisposition syndromes. Dr. Auber was in high school when… Read more »
Thank you to those who attended the recent webcast, “High Precision Exome CNV Detection with VS-CNV”. For those who could not attend but wish to watch, here is a link to the recording. This webcast delved into the complex world of CNV calling for whole-exome samples, which presents unique challenges that require specific considerations and strategies. Over the past several months,… Read more »
Researchers and clinicians alike benefit from the powerful capabilities of Golden Helix’s software. Our tools are continuously validated, and we like to showcase a few articles each month that demonstrate the multitude of use cases and advancements in science. For our April installment, I would like to highlight the clinical space, with users spanning the globe, all with a common… Read more »
I want to take this opportunity to highlight and briefly discuss some of the key features and updates that have been incorporated into VarSeq 2.2.3. Some of you may have attended the webcast that covered the prominent new features added to VarSeq, which are the updates to improve whole-exome analysis workflows, namely improved CNV calling in whole-exome datasets. However, there… Read more »
In many cases, VarSeq users design their next-gen sequencing workflows for a clinical application. One of the major values of using VarSeq is the standardization of sample analysis via project templates for filtering down to rare variants and isolate any clinically relevant variant. However, VarSeq also doubles as a robust research application as well. There are specific algorithms that can… Read more »
With the latest release of VarSeq, we have made significant updates to our handling of the interaction of variants and genes. This includes the support for non-coding transcripts, improved splice site predictions, and updates to gene and transcript annotations. We received several questions regarding how decisions are made in the software regarding genes and transcripts with these gene-related changes. This… Read more »
While VarSeq has always had excellent support for variant interpretation and analysis, we continue to find new edge cases in the clinical literature that improve our interpretation capabilities. In this blog, we will be covering some of the new improvements in VarSeq to support the interpretation of non-coding and splice site variants. Transcript Annotation Improvements Let’s start by covering some… Read more »
We are excited to share our latest publication with The Journal of Precision Medicine, “Implementing the ACMG Guidelines for CNV in a Commercial Software Solution”. “In 2020, ACMG in collaboration with the ClinGen working group developed a new set of guidelines for the clinical interpretation of CNVs. While theseguidelines provide a robust set of rules for interpreting intragenic deletions and… Read more »
Thank you to those who attended the recent webcast, “Exome Analysis with VS-CNV & VSClinical: Updated Strategies & Expanded Capabilities”. For those who could not attend but wish to watch, here is a link to the recording. In this webcast, we covered the capabilities and updates that have been incorporated into VarSeq that enhance whole exome sequencing workflows. The new… Read more »
The GenomeAsia 100K Variant Frequency database is a pilot annotation source now available to our users. This valuable database offers a deep characterization of specific populations in Asia that can be used to drive genetic studies. GenomeAsia is comprised of whole-genome sequencing data of over 1,000 individuals from 219 populations across Asia. Using this as an annotation, users can analyze… Read more »
Breakthroughs and discoveries in personalized medicine occur every day and here at Golden Helix, we are proud to play a role in so many cutting-edge investigations. It is always my pleasure to provide a brief description of what is only a small sample of our customer success stories over the course of four weeks and this month is no exception…. Read more »
In this blog, we will be covering new assessment catalogs and how they work to improve saving and tracking variant interpretations. VarSeq is a variant analysis tool that effectively analyzes single nucleotide (SNVs) and copy number variants (CNVs) in both cancer and germline workflows. Because VarSeq enables such diverse variant analysis, there are many research labs and institutions that evaluate… Read more »
Orphanet is a public database available in VarSeq that aims to improve the current understanding and treatment options for patients affected by rare diseases. This resource was established in France in 1997 and has gradually grown to cover a consortium of over 40 countries in Europe. The primary goal of this database is to provide a universal nomenclature to classify… Read more »
We would like to thank everyone who entered our 2021 Abstract Competition. It is wonderful to read about the different ways Golden Helix software is being applied around the world. It is our pleasure to announce this year’s winners! First Place: Viney Gupta – All India Institute of Medical Sciences – AIIMS Digenic Inheritance in Juvenile Open-AngleGlaucoma Sign up for… Read more »
Thank you to those who attended the recent webcast, “VSWarehouse: Tracking Changing Variant Evidence and Classifications”. For those who could not attend but wish to watch, here is a link to the recording. The webcast covered some general highlights of VSWarehouse value but also presented some specific capabilities covering the ClinVar classification tracker. Golden Helix provides complete solutions to handle… Read more »
Clinical testing labs produce reports as the end product of the NGS variant detection and interpretation workflow. Necessarily, the content, detail, and presentation of the report needs to be specialized to each clinical lab, and potentially each offered test. Our last blog post introduced the new Word-based report templates in VSClinical. In this blog post, we will introduce and explore… Read more »
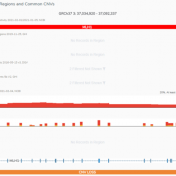
The collaboration between the Clinical Genome Resource (ClinGen) consortium and the American College of Medical Genetics (ACMG) recently developed published guidelines for the interpretation of CNVs called on next-generation sequencing data. These new guidelines are the first to provide a robust set of rules for the interpretation of small intragenic deletions and duplications and are now automated in VSClinical. … Read more »
This morning I released a new version of my eBook “Clinical Variant Analysis – Second Edition.” The clinical interpretation of variants in Next-Gen Sequencing is a quickly evolving field. While the body of knowledge is growing exponentially, experts have to derive sound, clinical decisions leveraging an ever-expanding set of specialty databases, clinical publications, and algorithms that are designed to predict the… Read more »
In many cases, VarSeq users typically run single trio projects or perhaps an extended family project. Not only are all the inheritance model algorithms available in the VarSeq software to capture de novo, dominant, or recessively inherited variants but there are a number of quality control fields to help ensure the pedigree was set up properly. The last thing any… Read more »
Next-generation sequencing generates an immense amount of data which is then subject to a multi-step process to establish a validated bioinformatic pipeline. From processing raw sequence data to the detection of genetic mutations, establishing a validated and consistent bioinformatic pipeline makes a huge difference in the quality of patient care and accuracy of results. In this blog, we are focusing… Read more »