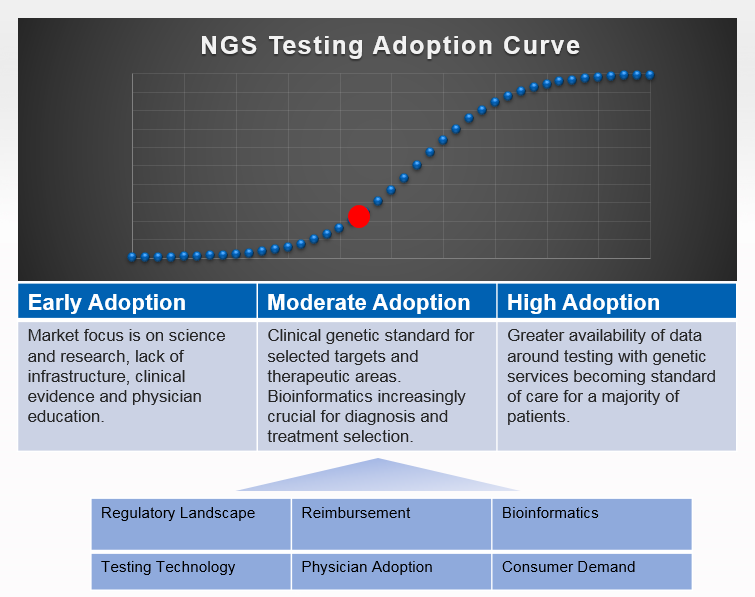
The adoption of genetic services is key to our ability to provide personalized medicine in the future. The goal is to better diagnose diseases, predict their outcome, and choose the best possible care option for a patient. We still have a long way to go to achieve this goal. While there is agreement about the ultimate goal, there is still a lot of uncertainty about the timing of the adoption. Essentially, it is a widely debated topic among experts. Here is what we know. There are three phases of the adoption (see also Fig. 1):
- Early Stage: Strong focus on science and research; understanding underlying genetic mechanisms and pathways.
- Moderate Adoption: Utilizing available results in the clinic on a selected basis. The science and clinical communities focus on increasing the number of therapeutic areas as well creating infrastructure to improve scale.
- High Adoption: At this point, genetic services are part of standard care.
There are a number of success factors that are key to drive adoption in the moderate phase of the curve:
- Regulatory Environment: This is a wide open field. The FDA is paying increasing attention to how genetic information about individuals is handled. Very publicly, it engaged with 23andMe to review their processes and procedures. While this gives the adoption process a pause in the short-term, it might mean that in the mid- and long-run, we can hope for a consolidated governing body protecting patients while enabling clinicians to make informed decisions.
- Testing Technology: There is already clinical experience leveraging genetic markers via gene panels. Many experts believe that the usage of Next-Gen sequencing data, including whole exomes and genomes, will ultimately give us the insights needed to diagnose or predict diseases and select treatment options at the highest level possible.
- Reimbursement: The adoption of these tests in the clinic hinges on the ability for doctors to recover the expense associated with genetic tests. For this to occur, the quality of the results of these tests has to be very high and their clinical utility must be proven. Also, price points have to become reasonable for payers and insurance companies to accept these tests as part of standard care…
If you wish to continue reading the eBook, I invite you to download a complimentary copy of my eBook: