If we take a look back in time, a lot happened in September 1998. It is the month in which the first ever “Who wants to be a Millionaire?” show debuted on ITV in Britain. Larry Page and Sergey Brin incorporated Google in September, registering the Google.com domain on September 15, 1998. And on that very same day, we officially formed Golden Helix, Inc. as a business entity! A few days later, the Internet Corporation for Assigned Names and Numbers (ICANN) was formed. The movie “Rush Hour” starring Jackie Chan and Chris Tucker was released. MCI Communications and WorldCom announced their mega-merger to form MCI WorldCom. And yes, the Human Genome Project was on its way – but it would take another four and a half years to be completed. As we gear up for the celebration of our 20th Anniversary next month, we would like to take a step back and reflect on how Golden Helix came to be one of the long-standing bioinformatics firms in our space.
While working for GlaxoSmithKline, Dr. Christophe Lambert had the idea to leverage genetic information to improve the drug development process. He was able to convince his employer to invest in this idea and chose Bozeman, Montana as the place to start the company. This was well-received and up to this date our employees love to work and live here. If you haven’t been to Montana, you can get an idea of why this is by taking a look at the photos below.
For the first 15 years, Golden Helix developed the industry-leading software solution, SVS. It is used by a wide range of research and government organizations to conduct Genome-Wide Association Studies, Genomic Prediction Analysis, as well as large-N case-control studies to research rare diseases among other things. Over this time, we obtained a deep understanding of the genomics space and created a powerful technology stack conducting advanced analytics on large genomic data sets.
In 2013, I joined the company as a CEO, member of the board and shareholder which allowed Christophe to pursue a successful career in academics. He is now a Professor with the Center of Global Health at the University of New Mexico. My goal was to lead the company into the clinical analytics space, leveraging its tremendous expertise and technical capability. We understood at the time that the whole market will grow beyond studying the correlation between genetic markers and diseases to the clinical application of this knowledge. We knew we had to develop new products to serve the evolving needs of our customers.
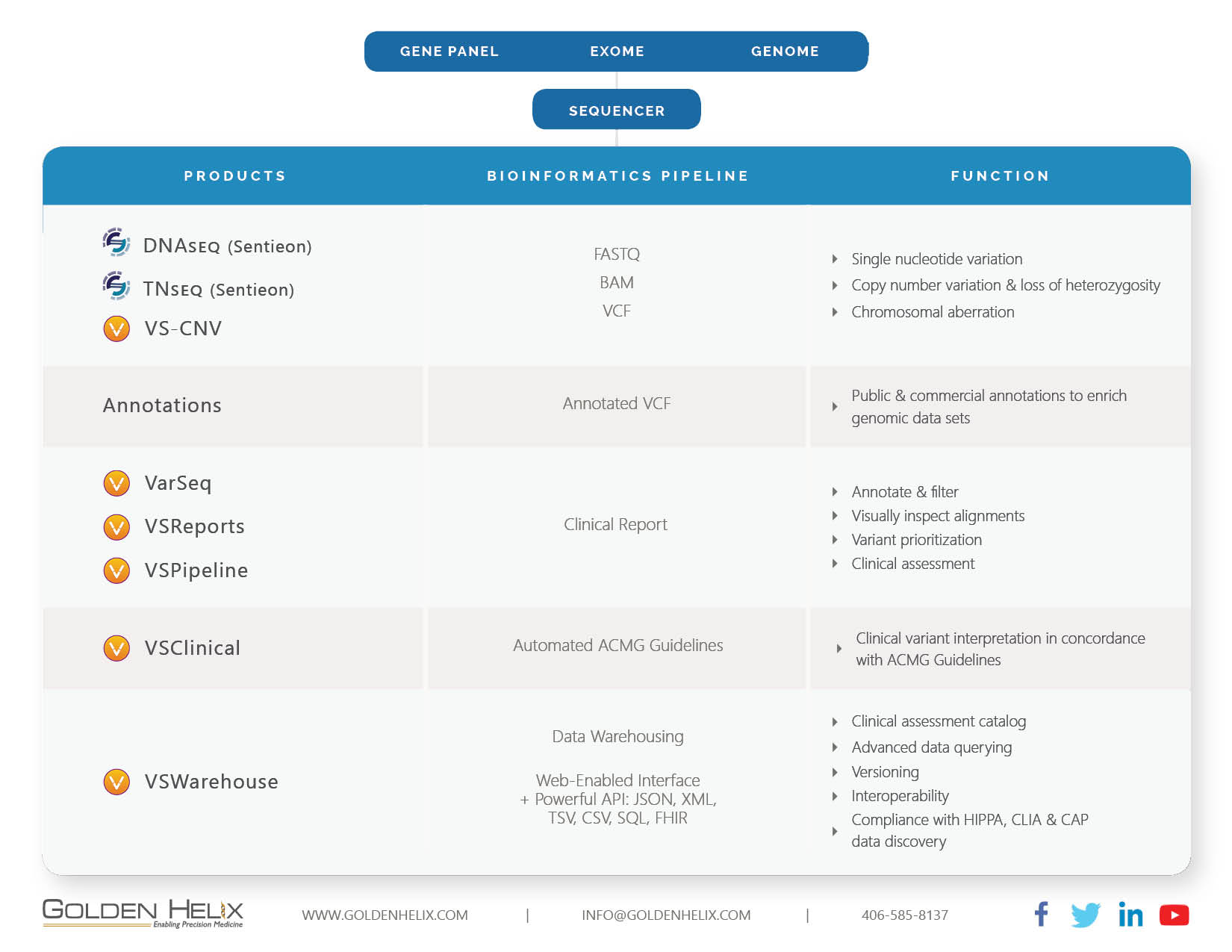
Genetic testing technology and infrastructure are evolving quickly. Hospitals and healthcare organizations around the globe are building the infrastructure necessary to handle the increasing testing volume. Over the last five years, Golden Helix has built a complete end-to-end bioinformatics pipeline that is designed to receive the data from the sequencer and take it all the way to clinical reporting. Alongside, we have created automation capability for high throughput labs, as well as an extensive data warehousing capability that allows the capture and querying of the entire lab output (see Fig 1). This complete end-to-end architecture sets Golden Helix apart in the marketplace. It allows our clients to conduct a thorough analysis of their sample data coming out of the sequencer. It supports the entire clinical interpretation and report generation. Lastly, it is able to store all data, make it retrievable and allows other hospital systems to connect to the data repository via APIs and standard protocols.
Secondary Analysis: Here we provide the unique ability to analyze genomic data in regards to Single Nucleotide Variations and Structural Variations. Via our partnership with Sentieon, we provide a highly performant secondary pipeline that includes alignment and variant calling on par with GATK and MuTect2 at much-improved speed levels. Our product VS-CNV is capable of detecting CNV events starting at the exon level all the way to aberrations of an entire chromosome.
Tertiary Analysis: VarSeq, VSClinical and VSReports are covering all clinically relevant workflows for the filtering and annotating of genomic data in concordance with applicable guidelines such as the ACMG. We support the analysis of gene panels, trios, single exome and whole genomes. With a single click, users can generate a clinical report that integrates the specific findings with annotation sources. There are powerful customization options available to make the reports exactly how your organization requires them to be. Moreover, with VSPipeline we have developed the ability to automate the entire pipeline to increase throughput. Our clients are able to automate the process from FASTQ to Clinical Report, including the computation of CNVs. This allows a highly efficient review of the resulting data. This not only saves time, but it also minimizes the potential for human error.
Data Warehousing: VSWarehouse captures the artifacts of the bioinformatics pipeline. Via powerful APIs, the product connects to other lab and hospital systems such as EPIC or Cerner’s Millennium. Moreover, it allows you to efficiently answer the following questions:
- Have I seen this variant before in my clinical practice? If so, was it included in any clinical report?
- Has the categorization of any variant that I reported on changed (e.g. from ‘unknown’ to ‘pathogenic’)?
- It allows you to version the clinical analysis conducted by lab work including the annotation sources that have been used during the tertiary analysis. This is a key capability during discovery should your lab or hospital be involved in legal disputes.
Our software stack is deeply integrated. This is a major advantage over any software architecture consisting of different point solutions of other commercial vendors or homegrown applications. Labs would have to carry the burden to integrate the various components via so-called ‘glue ware’, test the integration and maintain a detailed versioning log to stay in compliance with applicable regulatory requirements. In some cases, we are not even aware of any viable commercially available alternatives in areas such as our powerful CNV analysis product or our data warehousing solution. The high degree of integration brings enormous speed and performance advantages since we have been able to optimize data formats and leverage multi-threaded code components across the various products.
After 20 years of service, we are doing business with 400+ organizations globally. We can reference 1,200+ citations of researchers and clinicians using our software. CIO Review selected us as part of their “20 Most Promising Biotech Technology Solution Providers in 2017“. CIO Applications positioned us as the one of the “Top 25 Biotechnology Solution Providers in 2017” and Pharma Tech Outlook saw us among the “Top 10 Analytics Solution Providers” in 2018.
Above all of this, in an industry where things are fast changing, constantly evolving and companies have come and gone, we stand for longevity. Under our roof we have three generations of employees. Personally, I am proud to be the leader of this great organization with a great tradition and a fantastic future. To all our customers and partners, I’d like to extend our sincere ‘Thank you‘ for trusting us with your clinical work and research. It has been a huge honor to serve you for two decades. Together with you, we look forward to what the next 20 years will bring.







Nice advert – and happy 20 years
20 years and many more to go
Best wishes from your user down under – Val
Incredible evolution in a complex field! Way to go, Golden Helix!
Happy 20 years anniversary. Warmest wishes to the wonderful Goldenhelix and congratulations Andreas for such achievement